
guidebook
Nitrates in groundwater and Tapwater
A danger to our health?
Water quality is essential for our health. A critical aspect of this quality is the presence of nitrates in groundwater and Tapwater, which mainly originate from agricultural fertilizers and wastewater. Although nitrates occur naturally in small quantities, increased concentrations lead to health risks.
This Guidebook aims to raise awareness of the risks posed by high nitrate levels in water. We shed light on the causes and pathways of how nitrates enter our water, present the health risks and discuss what measures can be taken to minimize nitrate pollution. Our aim is to emphasize the importance of pure water sources and provide practical solutions for clean and safe water.
What are nitrates
Nitrates are chemical compounds consisting of nitrogen and oxygen, with the chemical formula NO3-. They occur naturally in the environment and are an essential part of the nitrogen cycle, a fundamental process that is crucial for plant growth and soil fertility. Nitrates play a central role in plant nutrition by providing an important source of nitrogen that plants can absorb and use for growth and development.
Despite their natural presence and role in the ecological system, nitrates can pose health risks to humans if they occur in excessive concentrations in water. These high concentrations are often caused by human activities, especially intensive agriculture. Fertilizers that are rich in nitrogen, as well as animal husbandry and improper waste disposal, can lead to an accumulation of nitrates in the soil. From there, they can leach into groundwater and eventually contaminate our water sources, including tap water.
In natural ecosystems, nitrates are usually in a balance that is regulated by processes such as denitrification, in which nitrates are converted back into gaseous nitrogen and released into the atmosphere. However, this balance can be disturbed by excessive nitrate inputs, which not only leads to a deterioration in water quality, but can also have ecological consequences such as excessive algae growth in water bodies.
In summary, nitrates are both a natural and necessary part of the ecosystem, but their concentrations are in some cases raised to harmful levels by human activities. A thorough understanding of the sources, pathways and effects of nitrates is crucial to protect water quality and ensure public health.

What is the difference between nitrate and nitrite
Nitrate and nitrite are both chemical compounds that contain nitrogen and oxygen, but they differ in the number of oxygen atoms and in their properties. Nitrates are beneficial to plants, while nitrites are more regulated due to their reactivity and potential health risks.
Nitrate properties
- Chemical formula: NO3-
- Contains one nitrogen atom and three oxygen atoms
- Occurs naturally in the soil and is an important nutrient for plants.
- In low concentrations, it is generally not harmful to humans.
- However, excessive nitrate intake can be problematic, as nitrates can be reduced to nitrites in the body.
Nitrite properties
- Chemical formula: NO2-
- Consists of one nitrogen atom and two oxygen atoms.
- Is more unstable and reactive than nitrate.
- Can be used in food as a preservative to inhibit the growth of bacteria.
- Can impair the oxygen uptake of the blood in the body, which can lead to health problems, especially in infants (blue baby disease).
- The formation of potentially carcinogenic nitrosamines is a hazard associated with nitrites.
GOOD TO KNOW!
In Germany, the limit value for nitrates in drinking water is set at 50 milligrams per liter by the Drinking Water Ordinance. This value applies to both adults and infants and is intended to ensure that there is no health risk from drinking water
Natural and anthropogenic sources of nitrates
Nitrates are found in our environment as a result of both natural processes and (anthropogenic) human activities. Understanding the main sources of this nitrate accumulation is crucial in order to take effective measures to minimize their impact on water quality and human health.
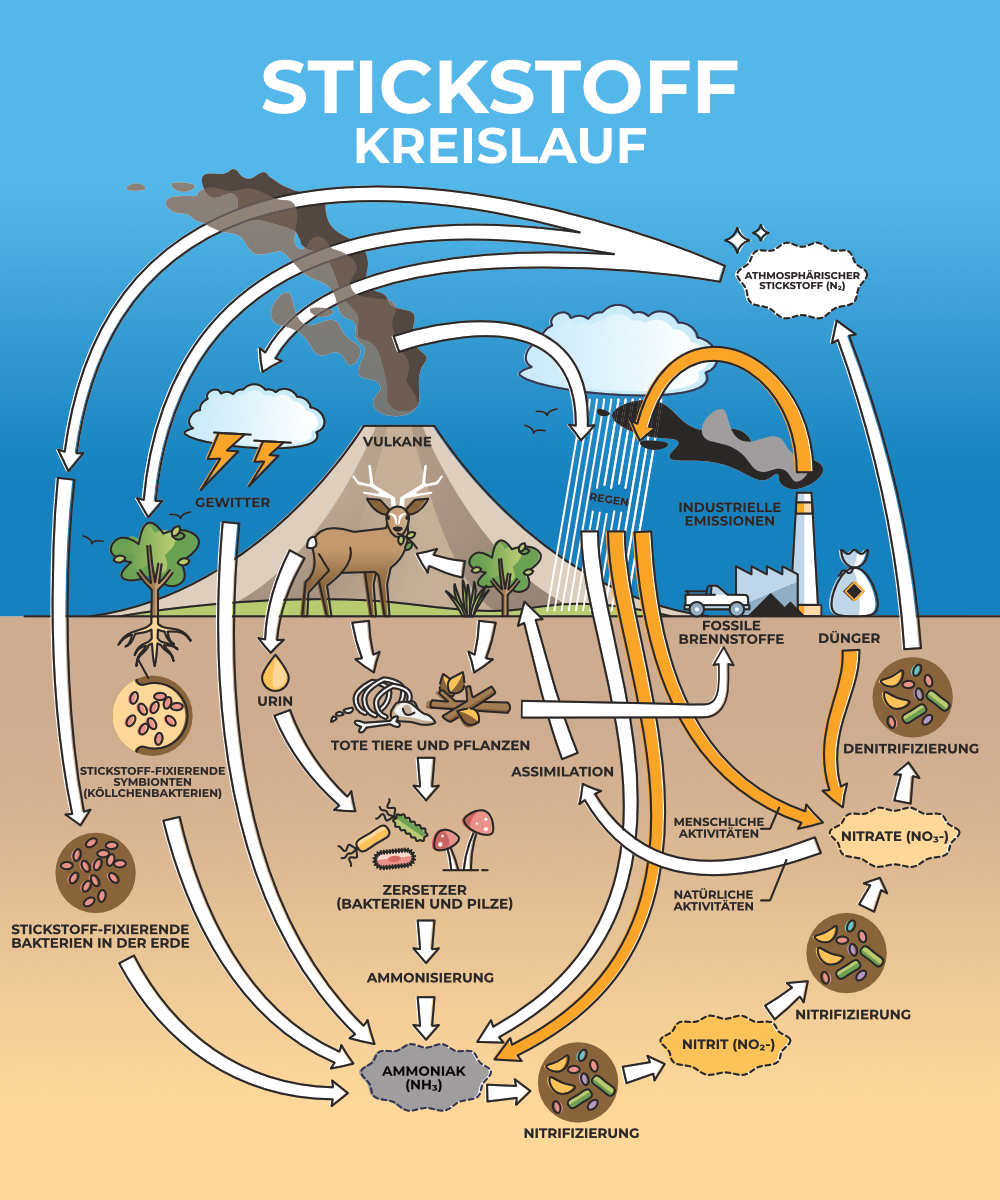
Natural sources of nitrates
- Nitrogen cycle: Nitrates are a major end product of the nitrogen cycle, a natural process in which nitrogen circulates in various forms between the atmosphere, the soil and living organisms. Microorganisms in the soil convert nitrogen compounds into nitrates through nitrification, which can then be taken up by plants.
- Atmospheric inputs: Nitrogen oxides (NOx), which are produced by lightning strikes or microbial processes in the atmosphere, can reach the earth as nitrates with precipitation.
- Decomposition of organic matter: The decomposition of plant and animal residues by microorganisms also leads to the release of nitrates into the soil and water.
Anthropogenic (human) sources of nitrates
- Agricultural fertilizers: The intensive use of nitrogenous fertilizers in agriculture is one of the main causes of increased nitrate concentrations in the soil and groundwater. Nitrates that are not absorbed by plants can leach into the groundwater and thus reach water sources.
- Livestock farming: Large quantities of slurry and manure from livestock farming also contain high levels of nitrogen. If these waste products are spread on fields in large quantities, the excess nitrate can enter the groundwater in a similar way to fertilizers.
- Industrial waste and wastewater discharge: Industrial processes and the discharge of untreated or inadequately treated wastewater into water bodies are further sources of nitrates. These inputs come from various industrial and municipal sources, including food processing and chemical production.
- Transportation and fossil fuel combustion: The combustion of fossil fuels in vehicles and power plants leads to the release of nitrogen oxides into the atmosphere, which can then enter the soil and waterways as nitrates through rainwater.
The combination of these natural and anthropogenic sources leads to the accumulation of nitrates in the environment. Increased awareness and targeted measures are needed to minimize the impact of these inputs on water quality and public health.
How do nitrates get into groundwater and Tapwater?
Nitrates enter the groundwater and Tapwater through various processes and pathways. A key process in this context is leaching, where water seeps through the soil, carrying soluble substances such as nitrates with it. These leached nitrates can then enter the groundwater, one of our primary water sources. The extent to which nitrates enter groundwater through leaching depends on several factors, including soil conditions, the amount of rainfall and the intensity of agricultural use in an area.

In regions with intensive agriculture, where large quantities of nitrogen fertilizers and animal waste are spread on the fields, nitrate pollution of the groundwater is often particularly high. The soil can only absorb a certain amount of nitrate that is needed by plants. Excess nitrates that are not absorbed by plants initiate the process of leaching, especially during rainfall or snowmelt, when the soil is saturated and can no longer absorb the water.
Another way in which nitrates enter the groundwater is through the infiltration of contaminated surface water. This can happen when rivers and lakes with high nitrate concentrations come into contact with groundwater or when contaminated rainwater infiltrates directly into the soil.
Urban and industrial sources also contribute to nitrate pollution. Inadequately treated wastewater that is discharged into natural bodies of water can infiltrate the groundwater. Similarly, nitrogen oxides from the air caused by the combustion of fossil fuels can enter the soil and groundwater through rainwater.
Once nitrates are in groundwater, they can travel long distances and contaminate large water supply systems. Since groundwater is often a major source for the Tapwater , nitrates eventually enter the domestic water supply. Treatment of Tapwater does not always remove nitrates effectively, especially in older or less advanced water treatment systems, emphasizing the need for careful monitoring and additional water treatment where necessary.
Legal regulations and limit values for nitrates
In Europe, the limit values for nitrates in water are set by several legal regulations in order to protect both the environment and human health. The most important of these are the EU Nitrates Directive and the EU Drinking Water Directive.
EU Nitrates Directive
The EU Nitrates Directive (91/676/EEC) was introduced in 1991 to reduce water pollution caused by nitrates from agricultural sources and to safeguard the quality of water resources. It obliges the member states to draw up and implement action programs to reduce nitrate pollution. This includes the identification of nitrate-sensitive areas (so-called "red areas") and the introduction of agricultural practices that minimize the input of nitrates into water bodies.
EU Drinking Water Directive
The EU Drinking Water Directive (2020/2184) defines the quality standards for drinking water within the European Union. A limit value of 50 milligrams per liter is specified for nitrates. This value is intended to ensure that drinking water does not pose a risk to human health, particularly with regard to the risks described above.
Drinking Water Ordinance (TrinkwV)
In Germany, the EU requirements are implemented by the Drinking Water Ordinance (TrinkwV) implemented. The TrinkwV adopts the limit value of 50 milligrams of nitrate per liter and stipulates that water supply companies must regularly monitor the quality of drinking water and report the results to the health authorities. If the limit is exceeded, water suppliers are obliged to take measures to restore the quality of the water and inform the public. In addition to the Drinking Water Ordinance, Germany also has the Groundwater Ordinance (GrwV), which also sets a limit of 50 mg/l nitrate for groundwater. If elevated nitrate levels are detected, the federal states are required to take appropriate measures to reduce nitrate inputs.
Nitrate pollution in Germany
Germany ranks second among the 28 EU member states when it comes to nitrate pollution in groundwater - surpassed only by Malta. Deutsche Umwelthilfe (DUH) criticizes the failure of German policy in this area for over a quarter of a century. It is calling for decisive measures to protect groundwater and drinking water affected by industrial agriculture.
According to the Nitrate Report 2020, 543 of the groundwater monitoring stations in Germany examined in 2012 exceed the limit of 50 milligrams of nitrate per liter. This corresponds to around 27% of all monitoring stations surveyed. In addition, 568 measuring stations, i.e. around 28%, have elevated nitrate levels of between 20 and 50 milligrams per liter. This data illustrates the challenges in dealing with nitrate pollution and the need for measures to improve water quality.
On the website of Proplanta.de there is an interactive map with the current values. Here you can follow the nitrate levels for each district in Germany over the last few years.
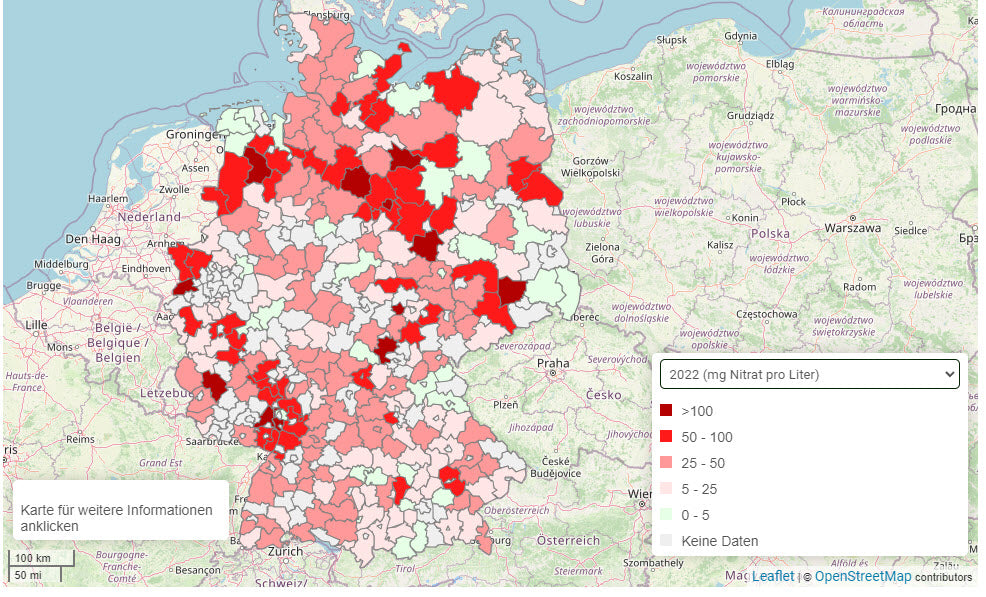
Map of Germany with nitrate pollution per district Source: Proplanta.de
10 districts with the highest nitrate pollution in Germany
| County | Measured values 2022 in mg/l | |
|---|---|---|
| Hildburghausen | Thuringia | 230,00 |
| Wolfsburg | Lower Saxony | 200,00 |
| Bad Dürkheim | Rhineland-Palatinate | 197,00 |
| Viersen | North Rhine-Westphalia | 153,48 |
| Lüchow-Dannenberg, | Lower Saxony | 141,90 |
| Erfurt | Thuringia | 112,31 |
| Celle | Lower Saxony | 111,81 |
| Rhine-Palatinate district | Rhineland-Palatinate | 111,05 |
| Meissen | Saxony | 104,6 |
| Cloppenburg | Lower Saxony | 104,07 |
Risks and effects of nitrates on our health
Nitrates in themselves are not particularly toxic and are usually processed safely by our bodies, especially when ingested through food. The main source of nitrate exposure for humans is the consumption of plant foods, but drinking water can also be a source, especially if it comes from a well with high nitrate contamination.The primary health concern regarding nitrates arises when they are reduced to nitrites in the body. This can happen:
- In the mouth through the action of bacteria.
- In the gastrointestinal tract, where nitrates can be reduced to nitrites under acidic conditions.
Nitrites can then impair the blood's ability to transport oxygen by converting hemoglobin to methemoglobin, which reduces oxygen transport. In adults, methemoglobin is usually quickly converted back to hemoglobin, but in infants, especially those under six months of age, this process is less efficient. This can lead to methemoglobinemia, also known as blue baby disease, in which the skin may appear bluish due to lack of oxygen.
However, the main concern is the formation of nitrosamines, which can occur when nitrites react with amines and amides in stomach acid. Nitrosamines are carcinogenic substances that are associated with an increased risk of stomach and bowel cancer.
The effect of nitrates on human health is therefore a complex issue that encompasses not only the direct effects, but also the indirect risks through transformation products and their interactions in the body.
The exact effects and the threshold above which nitrates are harmful to health can vary from individual to individual and depend on numerous factors such as individual health, diet and general lifestyle. For these reasons, it is important to monitor and maintain nitrate levels in drinking water and food in order to protect public health.
Methods for monitoring and analyzing nitrate levels
Various methods are used to analyze nitrate levels, including laboratory tests that use chemical reactions to measure the concentration of nitrates, as well as simpler methods such as test strips that show color changes to quickly assess nitrate levels and are also used by private individuals due to their ease of use.
Modern technical processes such as ion chromatography offer precise and detailed analysis options. Electrochemical sensors are used for continuous measurements. These methods are used to check compliance with limit values and contribute to the protection of human health and the environment.
Solutions and measures to reduce nitrate pollution
There are several effective approaches and measures to reduce nitrate pollution in water resources. By combining the following measures, nitrate pollution can be reduced and water resources protected:
- Changing agricultural practices
One key measure is to optimize the use of fertilizers in agriculture. This can be achieved by using less nitrogen fertilizers, using alternative organic fertilizers or by fertilizing more precisely according to the actual needs of the plants. - Irrigation management
Efficient water management can reduce the leaching of nitrate into the groundwater. - Plant selection and crop rotation
Growing plants that use nitrogen efficiently and sensible crop rotation planning can help to retain nitrogen in the soil and prevent it from leaching out. - Enrichment strips and buffer zones
The creation of plant strips along waterways can act as a filter for water run-off from arable land and thus minimize nitrate inputs into surface waters. - Water treatment technologies
In areas with high nitrate contamination, water treatment systems can be used that are specially designed to remove nitrates from drinking water, for example by ion exchange or reverse osmosis. - Regulatory measures
Pollution can be controlled by laws and regulations, such as fertilizer ordinances, which set an upper limit for nitrate inputs. - Monitoring and education
Regular monitoring of nitrate levels in water sources and educating farmers and the public about the effects and management of nitrates are important for the long-term reduction of nitrate pollution.
FAQs on nitrates in groundwater and drinking water
Related articles and products
Sources
- dbgw.de - Nitrate in water
- Nitrate report of the Federal Environment Agency
- Federal Environment Agency - Nitrate report 2020
- business-biodiversity.eu - Second-highest nitrate pollution of groundwater in the EU
- proplanta.de - Nitrate pollution in groundwater
- Wikipedia.org - Nitrates
- eur-lex.europa.eu - Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption





